
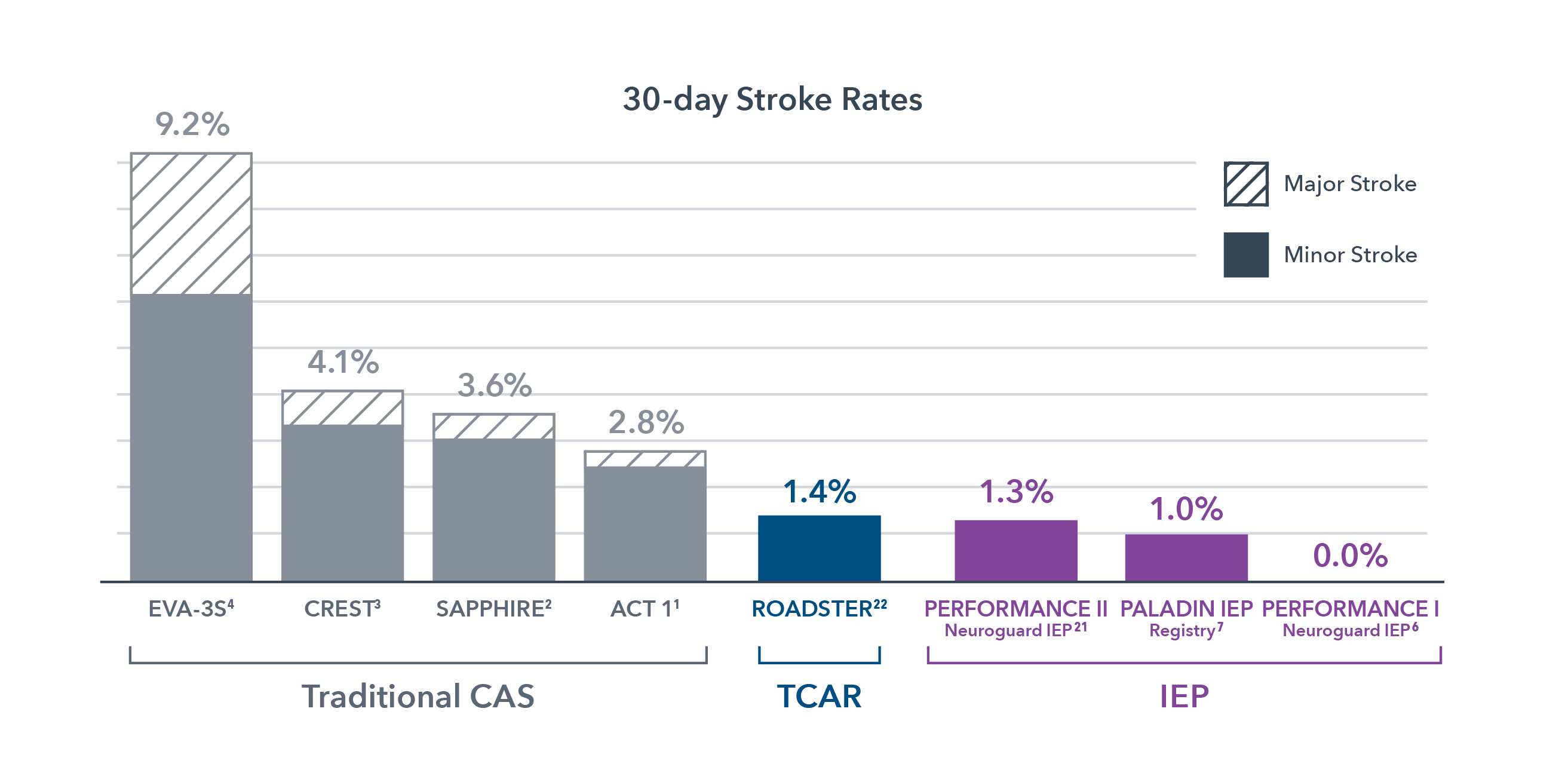
When it comes to carotid stenting, details matter. Time matters. And above all, safety matters. Historically, clinical studies of carotid stenting (CAS) show stroke occurs in 3-9% of patients.1-4 Most strokes are due to embolization during stenting and balloon dilation in the index procedure5, resulting in poor patient outcomes and significantly increased hospital costs.


The Neuroguard IEP System is approved for sale in the USA. Caution: Federal (United States) law restricts this device to sale by or on order of a physician. Prior to use, please see the Instructions for Use for a complete listing of Indications, Contraindications, Warnings, Precautions, Potential Adverse Events, Operator Instructions, and Directions for Use.
1ACT 1: N Engl J Med 2016;374:1011-20.
2SAPPHIRE Randomized: Yadav JS, et al. N Engl J Med 2004;351:1493-501.
3CREST: Brott et al, N Engl J Med 2010;363:11-23.
4EVA-3S: Mas, Jean-Louis, et al. N Engl J Med 2006;355:1660-71.
5Obeid, et al. J Vasc Surg. 2015 Sep;62(3):616-23.
6Zhou W, et al. Jour of Vasc Surg. 2017 Mar;65(3):686-694.
7Hitchner E, et al. Jour of Vasc Surg. 2016 Dec;64(6):1719-1725.
8Maggio P, et al. Jour of Neuro Sciences. 2013 May 15;328(1-2):58-63
9Per IFUs of devices: Pore sizes of various embolic protection devices: Angioguard 100µm, NAV6 120µm, and mesh-covered stents: CGuard 165µm, Terumo 375µm, Gore 500µm.
10Langhoff R, et al. Catheter Cardiovasc Interv. 2022;100(6):1090-1099.
11Langhoff R, et al. JACC Cardiovasc Interv. 2019;12:395-403.
12Gray W, et al. JACC Cardiovasc Interv. 2025;18(3)367-376
13Kwolek, CJ et al. J Vasc Surg. 2015 Nov;62(5):1227-34.
© 2025 Contego Medical, Inc. All rights reserved. MKT-1374
Paladin, Neuroguard IEP, Vanguard IEP, Excipio, Integrated Embolic Protection, and FlexRing are trademarks or registered trademarks of Contego Medical.